Toxicological Effects of Nanoparticles on Cells
Pt nanoparticles
Pt nanoparticles might enter the food chain, e.g. as a contaminant on the surface of food plants. However, there is only little knowledge about their uptake into cells of the gastrointestinal tract. Therefore, it is of general interest to study the correlation between particle properties like size, shape, and crystal structure on the one hand and biological responses on the other.
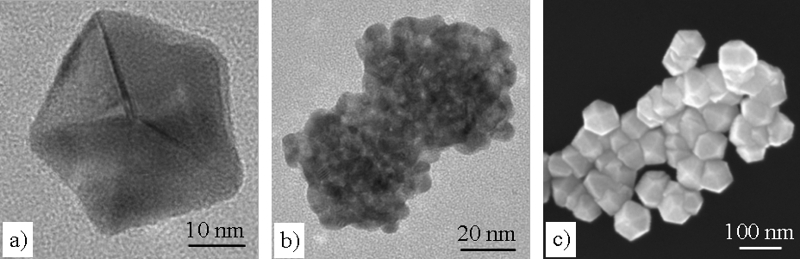
Human colon carcinoma cells (HT29) were chosen as a model system for the study of the uptake of Pt nanoparticles. In order to control the particle properties, Pt nanoparticles are produced on β-cyclodextrin (β-CD) by supercritical fluid reactive deposition (SFRD) in the group of M. Türk (Institut für Technische Thermodynamik und Kältetechnik, Karlsruhe Institut für Technologie (KIT)/Universität Karlsruhe). Depending on the organometallic precursors and process conditions, SFRD enables the deposition of pure and highly dispersed Pt nanoparticles with distinctly different sizes and shapes. This is illustrated in the electron microscopic images below, showing as examples a single decahedral nanoparticle with about 40 nm size (a), an agglomerate of very small (~5 nm) irregularly-shaped particles (b), and approximately 100 nm sized Pt particles with predominantly cuboctahedral shape in Fig. (c).
he cellular uptake and biological response is studied in dependence of the mean particle size, particle concentration, and time of incubation in the group of D. Marko (Institut für Analytische Chemie und Lebensmittelchemie, Universität Wien). A significant damage of the DNA is observed for small nanoparticles with sizes below 30 nm.
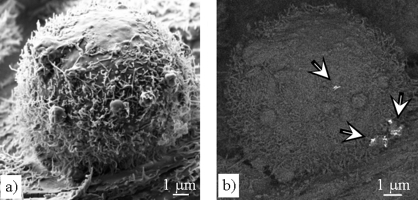
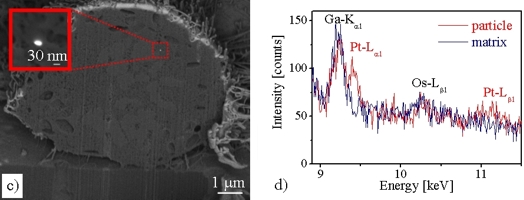
Pt nanoparticles in the cells can be directly visualized by using scanning electron microscopy (SEM) and focused ion-beam (FIB) milling HT29 cells. The cells are grown on Transwell membranes and are prepared for the SEM/FIB studies by fixation, dehydration and critical-point drying. Fig. (a) below shows a secondary-electron (SE) image of a single HT29 cell after 24 h Pt (1 μg/cm2) incubation. The surface of the HT29 cell is characterized by microvillis and some phagocytotic protrusions. In the backscattered-electron (BSE) image of the same cell (Fig. (b) below) agglomerates of Pt particles are revealed on the cell surface (arrows). This HT29 cell was subsequently cut “slice-by-slice” with the focused Ga+-ion beam in the FIB instrument and imaged by BSE. In this way, Pt particles were found in different depths in the cell interior (cf. Fig. (c) below) demonstrating cellular uptake. Moreover, the presence of platinum was detected by energy-dispersive X-ray spectroscopy (EDXS) as demonstrated in the EDXS spectrum (d) below.
SiO2 nanoparticles
SiO2 nanoparticles are used in the cosmetics and food industry as additive, e.g. to improve the rheological properties of products. Therefore, it is of general interest to study the effects of SiO2 nanoparticles on cells.
Human colon carcinoma cells (HT29) were grown on Transwell® membranes and incubated with commercially available SiO2 nanoparticles for 24 hours. After incubation, the cells were prepared by fixation with glutaraldehyde and osmium tetroxide, followed by dehydration and embedding in epoxy resin. Cell sections produced by conventional ultramicrotomy were investigated in a ZEISS CrossBeam 1540 EsB microscope by high-angle annular dark-field scanning transmission electron microscopy at 30 kV.
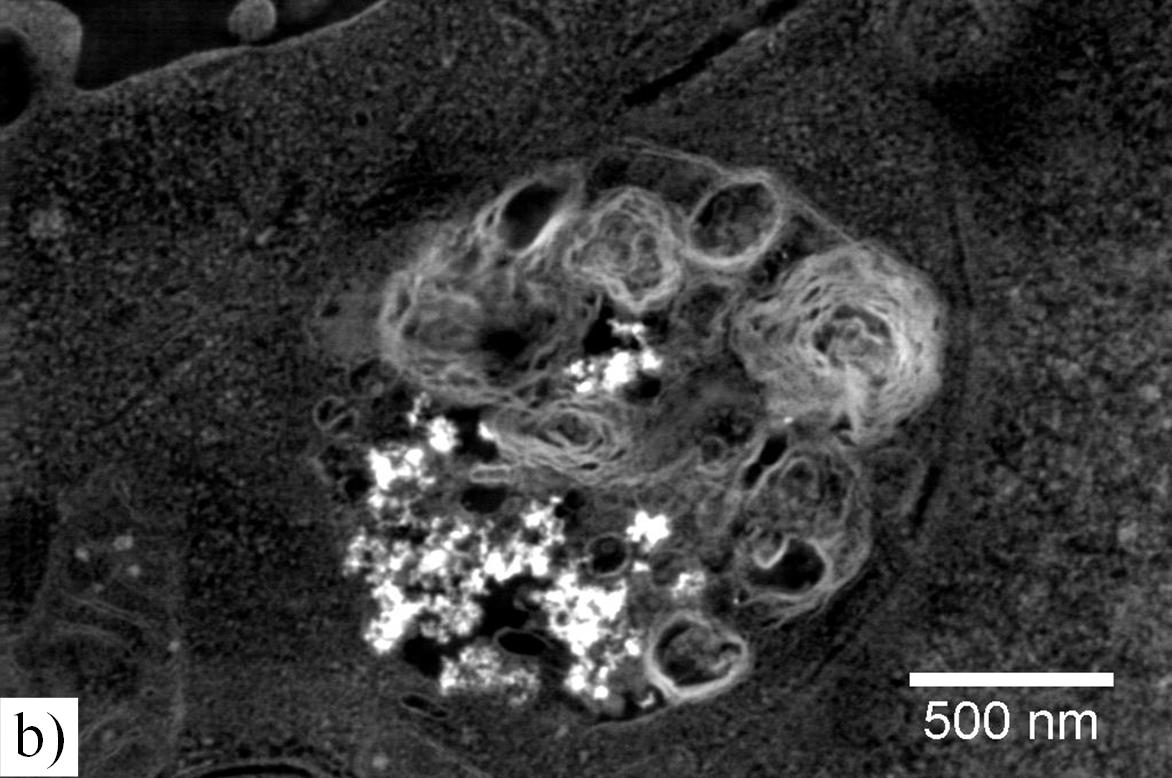
Using this method, SiO2 particles could be easily identified due to their bright contrast with respect to the cell matrix as demonstrated in Fig. (a). Moreover, cell organelles could be well recognized in these unstained samples. It is clearly shown that nanoparticles enter the cells. Lamellar bodies (Fig. (b)), vesicles and the cytosol can be associated with the locations of the particles.

1] J. Pelka, H. Gehrke, M. Esselen, M. Türk, M. Crone, S. Bräse, T. Muller, H. Blank, W. Send, V. Zibat, P. Brenner, R. Schneider, D. Gerthsen, D. Marko, Cellular Uptake of Platinum Nanoparticles in Human Colon Carcinoma Cells and Their Impact on Cellular Redox Systems and DNA Integrity,
Chemical Research in Toxicology 22 (4), 649-659 (2009) ![]()
[2] H. Gehrke, J. Pelka, C.G. Hartinger, H. Blank, F. Bleimund, R. Schneider, D. Gerthsen, M. Türk, D. Marko, Platinum nanoparticles and their cellular uptake and DNA platination at non-cytotoxic concentrations,
Arch. Toxicol. 85, 799 (2011) ![]()
[3] H. Gehrke, A. Frühmesser, J. Pelka, M. Esselen, L. Hecht, H. Blank, H. P. Schuchmann, D. Gerthsen, C. Marquardt, S. Diabaté, C. Weiss, D. Marko, In vitro toxicity of amorphous silica nanoparticles in human colon carcinoma cells,
Nanotoxicology 7(3), 274 (2013) ![]()
[4] H. Blank, R. Schneider, D. Gerthsen, H Gehrke, K. Jarolim, D. Marko, Application of low-energy scanning transmission electron microscopy for the study of Pt-nanoparticle uptake in human colon carcinoma cells,
Nanotoxicology Vol 8, No4, 433-446 (2014)
[5] H. Blank, R. Schneider, D. Gerthsen, H. Gehrke, K. Jarolim, D. Marko Application of low-energy scanning transmission electron microscopy for the study of Pt-nanoparticle uptake in human carcinoma cels,
Nanotoxicology 8, 433 (2014)
